Explain Why H2o and Co2 Molecules Have Different Shapes.
The molecular geometry of NH 3 is called trigonal pyramidal see Figure 9. In carbon dioxide there are no unshared pairs so the bonds are on opposite sides of the carbon atom forming a linear shape.
Why Is Co2 A Salient Linear Molecule But H2o Is Not Quora
On the other hand carbon dioxide is linear.
. This works well for very simply molecules like H2 Li2 and CO2 that have a small number of. In the ammonia molecule one of the electron pairs is a lone pair rather than a bonding pair. Click hereto get an answer to your question Although both CO2 and H2O are triatomic molecules the shape of H2O molecules is bent while that of CO2 is linear.
Carbon dioxide has a linear geometry because the lone pair and bond pair repulsion cancels out. The reason water has a bent shape is that the two lone pair of electrons are on the same side of the molecule. Water is a bent molecule due to the lone pairs present in oxygen.
Each CO bond. The lone pairs push the hydrogen atoms creating a bent shape. VSEPR theory is used to explain the 3D shape around an atom in a molecule and for small molecules this can give the overall shape.
Carbon dioxide is a linear structure with two double bonds between carbon and oxygen. In H2O the central oxygen has two free electron pairs attached to it. Explain this on the basis of dipole moment of the significanceapplications of dipole moment.
Hence it is a gas. In CO2 however there are two double bonds and no lone electrons on the central atom hence the molecule has a linear shape. Asked Dec 21 2020 in Chemical Bonding by Taashi 158k points chemical bonding.
Now we just have to decide whether ceH2O or ceH2S has a smaller bond angle. The central carbon in CO2 does not have any free electron pairs since all its electrons are in the two double bonds. In carbon dioxide the carbon is flanked by the oxygen atoms OCO.
The reason why carbon dioxide is a gas and silicon dioxide is a solid is because their chemical structures are different. CO 2 molecule has only two bonds which lie farthest to each other to minimise repulsion making an angle of 180. These pairs take up space forcing the two oxygen-hydrogen bonds to bend away from them.
This is due to the different numbers of electrons in each molecule and VSEPR Valence Shell Electron Repulsion theory. Hence it is a gas. In a water molecule there are two lone pairs of electrons connected to the oxygen.
They really repel each other as they are only attracted to the oxygen atom in the water molecule. Carbon dioxide is linear OCO so the bond polarities cancel. This repulsion of the lone pairs of electrons on the oxygen atom causes the bond of the hydrogen to the oxygen to be pushed.
Explain this on the basis of dipole. The domain geometry for a molecule with four electron pairs is tetrahedral as was seen with CH 4. H2O is polar as the molecule is bent and contains a dipole.
Why do water molecules have bent shape and carbon dioxide molecules have a linear shape. -CO2 does not have polar bonds-Oxygen has a different attraction for electrons in H2O than in CO2-These compounds have different shapes. Lone pairs of electrons play a significant role in the shape of molecules but it is not.
Use VSEPR theory to explain why. The key lies in the shape of the molecules. In water molecules the electrons on the oxygen atom form a tetrahedral shape which gives the three atoms of water.
Why is CO2 a linear molecule whereas H2O has a v-shaped geometry. H2O consists of polar molecules. Both the O-H and the CO bonds are polar but the arrangement of the atoms is different.
For large molecules such as proteins the overall shape will be due to the bonding between atoms as well as other electrostatic and steric effects. It is an oddity. Therefore we expect ceSO2 to have the largest bond angle of the four molecules and this is indeed the case.
This theory states that as electrons are negatively charged the valence electrons in different atoms in a molecule repel each other. Explain why different molecules have different sequences of energy in their molecular orbitals. How do chemists explain the difference.
As lone pair- bond pair repulsion is greater than bond pairbond pair repulsion the repulsions due to the lone pair decreases the angle between the two bonds giving the molecule a bent shape. Explain why hydrogen fluoride HF and water H2O are very polar molecules but CF4 and CO2 are nonpolar HF is polar as the molecule only contains two atoms with different electronegativity values. Although both CO 2 and H 2 O are triatomic molecules the shape of H 2 O molecule is bent while that of CO 2 is linear.
However water has a bent structure because only the oxygen atom possesses a lone pair which brings the bonding electron pairs closer. CO2 consists of nonpolar molecules. Carbon dioxide is a linear structure with two double bonds between carbon and oxygen.
Although both CO2 and H2O are triatomic molecules the shape of H2O molecule is bent while that of CO2 is linear. It is a small molecule and non-polar with only weak bonds between the molecules. The lone pairs push the hydrogen atoms creating a bent shape.
CeH2O and ceNH3 are hydrides of the same period so we can use the first rule to determine that ceH2O has a smaller bond angle. Molecules of carbon dioxide and water have different shapes even though they both have three atoms. In CO2 however there are two double bonds and no lone electrons on the central atom hence the molecule has a linear shape.
The reason why carbon dioxide is a gas and silicon dioxide is a solid is because their chemical structures are different. It is a small molecule and non-polar with only weak bonds between the molecules. H 2 O on the other hand has a lone pair.
Similar H2o Consists Of Polar Molecules Co2 Consists Of Nonpolar Molecules How Do Chemists Explain This Difference Quora
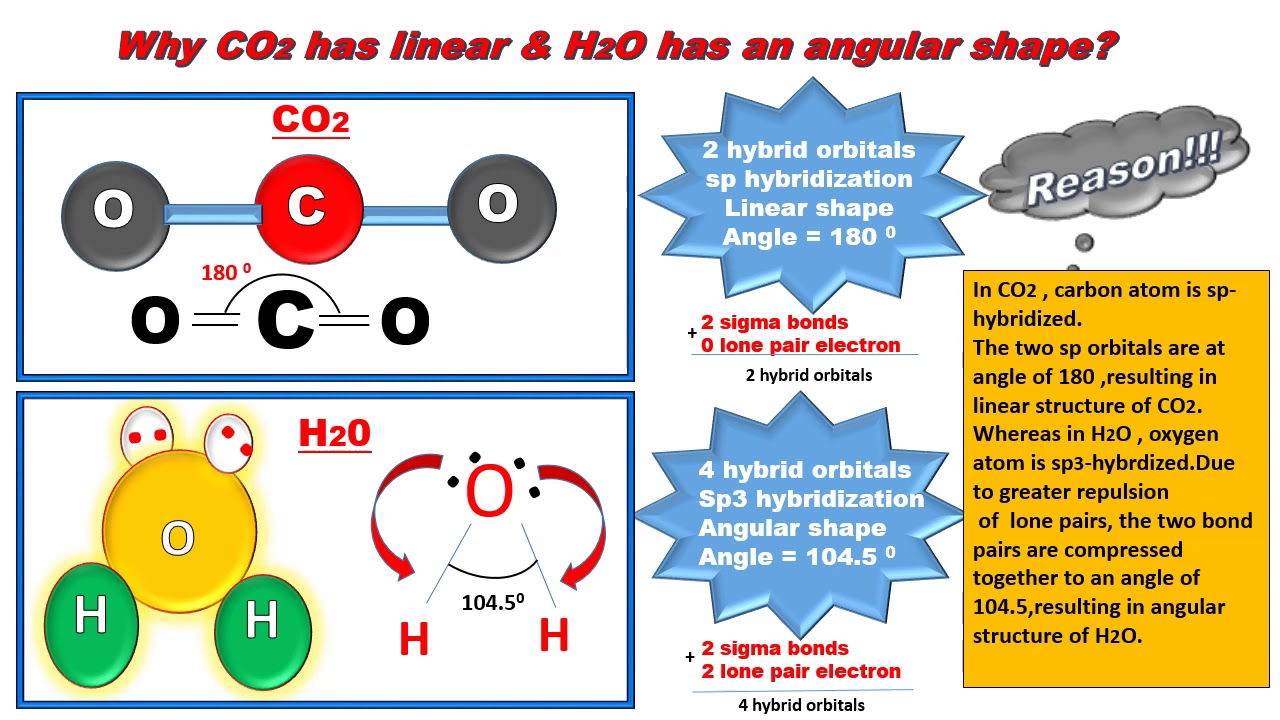
Why Water Has Bent Structure Whereas Carbon Dioxide Is Linear Hybridization Tips And Tricks Youtube
Carbon Dioxide And Water Molecules Both Have Three Atoms However Carbon Dioxide Is Linear While Water Is Bent How Do You Account For The Difference In Their Molecular Geometries Quora
Comments
Post a Comment